On October 12, 2018, the EU commission published Regulation (EU) 2018/1513, adding Entry 72 into annex XVII of REACH to restrictthe use of 33 substances classified as carcinogenic, mutagenic or toxic for reproduction(CMR), category 1A or 1B in clothing, related accessories, other textiles and footwear products. These restricted CMR substances and their limits are listedin appendix 12. The new amendment sets a general two-year transitional period, and will come into force on November 1, 2020.

The highlights of the amendment are as follows:
Scope:
The CMR substances listed in appendix 12 shall apply to the following products for use by consumers:
a.Clothing or related accessories.
b.Textiles other than clothing which, under normal or reasonably foreseeable conditions of use, come into contact with human skin to an extent similar to clothing.
c.Footwear.
Exemption:
a.Clothing, related accessories or footwear, or parts of clothing, related accessories of footwear made exclusively of natural leather,fur or hide.
b.Non-textile fasteners and non-textile decorative attachments.
c.Second-hand clothing, related accessories, textiles other than clothing or footwear.
d.Wall-to-wall carpets and textile floor coverings for indoor use, rugs and runners.
e.Personal protective equipment within the scope of Regulation (EU) 2016/425 and medical devices within the scope of Regulation(EU) 2017/745.
f.Disposable textiles (means textiles that are designed to be used only once for a limited time and are not intended for subsequent use for the same or a similar purpose)
Appendix 12 (33 restricted substances):
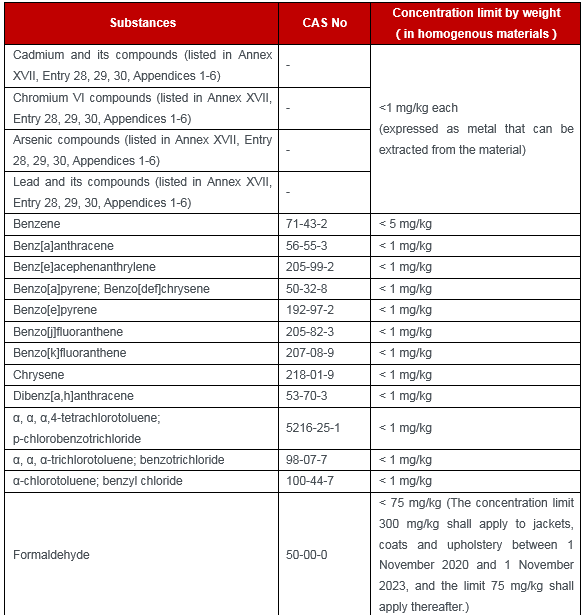
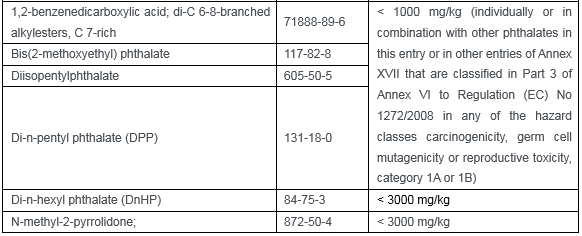
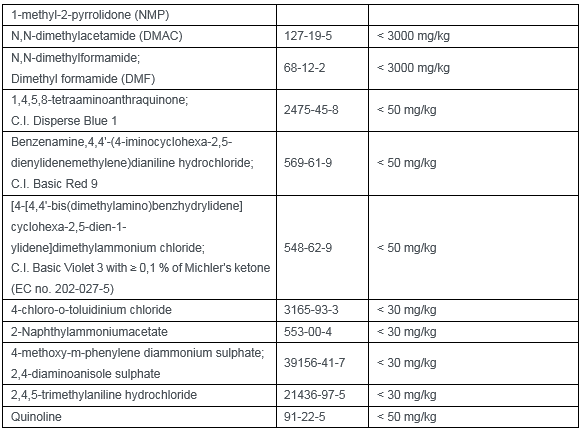
This amendment has a significant impact on Chinese textile, apparel and footwear enterprises. If the CMR substances mentioned above are used to relevant products that export to EU, enterprises shall verify the products and materials during the transition period and to ensure the compliance of products in advance.
Reference
https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1540172923880&uri=CELEX:32018R1513
