On August 11, 2025, the European Commission formally enacted a new regulation, (EU) 2025/1731, amending the list of CMR (carcinogenic, mutagenic, or toxic to reproduction) substances in the appendices of Annex XVII to the REACH Regulation ((EC) No 1907/2006), and adding an exemption for cumene in Appendix 11. The new requirements will officially take effect across the European Union on September 1, 2025.
The regulation includes the following two key amendments:
Revision of Appendices 2, 4, and 6: A total of 16 new CMR Category 1B substances have been added to Appendices 2, 4, and 6 to synchronize with the latest updates under the CLP Regulation.
(1)Limit requirements for the 16 newly added substances:
In accordance with Articles 28, 29, and 30 of Annex XVII to the REACH Regulation, mixtures containing any of the 16 newly added CMR substances at concentrations exceeding the limits specified in the EU CLP Regulation ((EC) No 1272/2008) will be prohibited from being placed on the EU market.
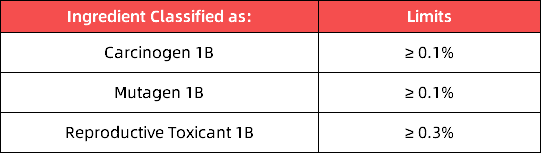
(2)List of 16 Newly Added Substances:
a.in Appendix 2, the following entries are inserted in the table in the order of the index numbers set out therein:
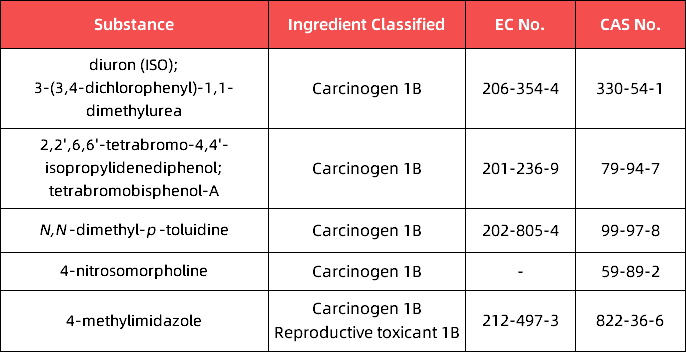
b.in Appendix 4, the following entry is inserted in the table in the order of the index numbers set out therein:

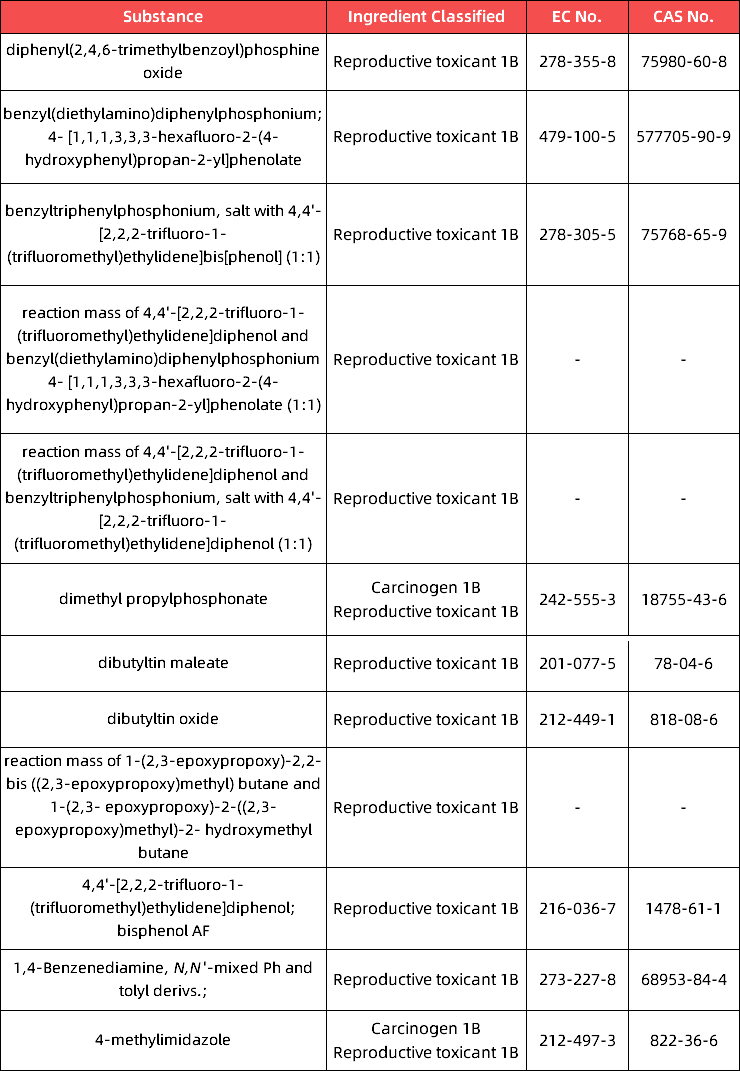
c.in Appendix 6, the following entries are inserted in the table in the order of the index numbers set out therein:
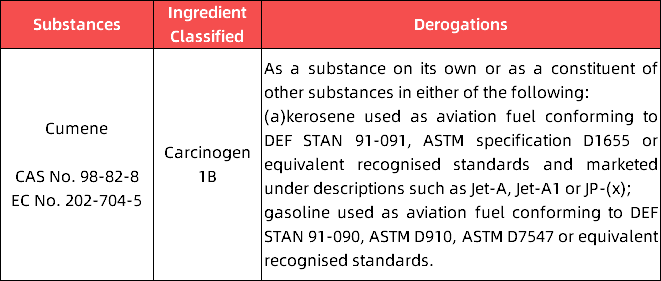
(3)in Appendix 11, the following entry is added:
CTT Reminder:
Companies exporting to the EU should closely monitor these updated requirements and implement necessary improvements in advance. CTT offers extensive product testing expertise to help you easily determine whether your products are safe and compliant. Feel free to consult with us for assistance.
Link:https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L_202501731
